Mitte Home.
Macht Wasser lecker reiner sprudelnd besser.Mitte Wasser-Abo
Ein Monat unverbindlich testen für nur 9,99 €

Doppelter Filter

Mineralisierung nach Wahl

Variable Sprudeloptionen

Bequem im Wasser-Abo
.svg)


Mehr als ein
Wassersprudler.
Mitte Home filtert, mineralisiert und sprudelt dein Leitungswasser auf Knopfdruck.
Entwickelt und hergestellt in Deutschland.
Lerne das Gerät kennen
Mehr als ein
Wassersprudler.
Mitte Home filtert, mineralisiert und sprudelt dein Leitungswasser auf Knopfdruck.
Entwickelt und hergestellt in Deutschland.
Lerne das Gerät kennenSo funktioniert es:
Macht Wasser reiner.
Mach dir keine Sorgen mehr über Mikroplastik, Schwermetalle, Chemikalien oder Bakterien im Leitungswasser.
Die Mitte Home-Kartusche filtert und reduziert viele der in deinem Wasser vorkommenden Schadstoffe.


Macht Wasser lecker.
Warum schmeckt Wasser aus der Leitung nie so gut wie natürliches Quellwasser? Weil die Mineralstoffe fehlen.
Daher lässt Mitte Home dein Wasser auch durch Schichten von Mineralgestein laufen. Mit verschiedenen Kartuschen für unterschiedlich Geschmack.
Macht Wasser still oder sprudelnd.
Wähle aus, mit wie viel Kohlensäure du dein Wasser am liebsten trinkst – ganz still, sanft prickelnd oder herrlich sprudelnd.

Bequem genießen ab
0,39 € pro Liter.
Erhalte ein Leihgerät und lass dir laufend neue Kartuschen nachliefen – schon ab 29,99 € im Monat.
Günstiger. Aber genauso gut wie Premium-Mineralwasser vom Getränkemarkt.
Nachhaltiger. Denn Einweg-Plastikflaschen und lange Treansportwege werden vermieden.
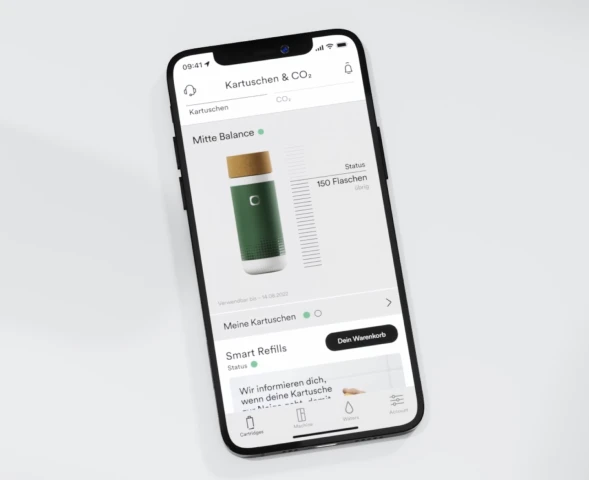
Ohne Kistenschleppen. Praktische Kartuschen- & CO₂-Nachbestellungen in der App, passend zu deinem Wasserbedarf. Bequem. Stressfrei.

Wasser-Abo: So funktioniert es:
Macht Wasser besser.
Mit Mitte Home bekommst du den leckeren Geschmack von Premium-Mineralwasser, die Nachhaltigkeit und Gesundheit von Wasserfiltern und den Komfort eines Wassersprudlers – alles in einem Gerät.

Macht Wasser günstiger.
Um täglich 1,5 l Wasser zu trinken, benötigst du ca. 500 l im Jahr.
Im Vergleich zu einem ebenso erfrischenden und gesunden Premium-Mineralwasser kannst du schon im Single-Haushalt bis zu 300 € im Jahr sparen.
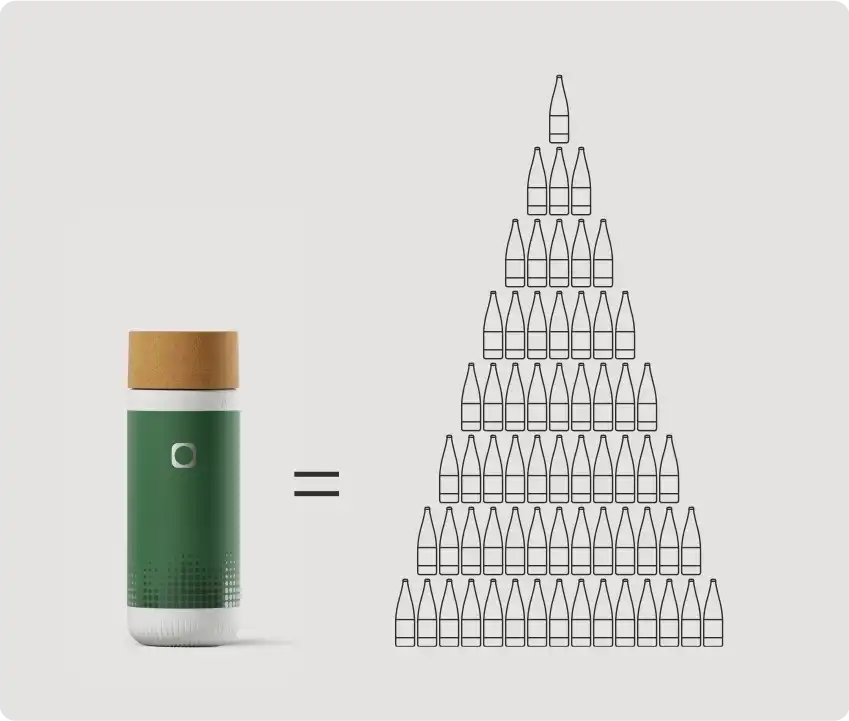
Macht Wasser nachhaltiger.
Eine einzige Mitte-Balance-Kartusche kann bis zu 300 Einwegflaschen Wasser ersetzen und erspart der Umwelt so 90 kg CO₂-Emissionen.
Erfahre mehr zu NachhaltigkeitMitte Home im Vergleich
Macht Wasser günstiger
Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.

Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.

Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.

Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.

Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.
Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.
Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.
Das Mitte Wasser-Abo: Günstiger. Bequemer. Besser.
So funktioniert das Abo:
Zu Beginn schicken wir dir das Mitte Home-Starterkit. Es enthält:
1 Mitte Home-Leihgerät:
Dein Wasseraufbereiter
1 Mitte Balance-Kartusche
zum Filtern und Mineralisieren
1 CO₂-Zylinder
zum Sprudeln & besserer Aufnahme der Mineralien
Wasserflasche(n)
860 ml, aus Glas und/oder PET
Danach senden wir dir regelmäßig eine neue Kartusche zu – du bestimmst, wie oft.
CO₂ zum Sprudeln kannst du selbst je nach Bedarf in unserem Shop nachbestellen – immer rechtzeitig mit der Smart Refills-Funktion.
Nach Ende deines Abos sendest du uns das Gerät wieder zurück.
So funktioniert das Abo:
Zu Beginn schicken wir dir das Mitte Home-Starterkit. Es enthält:
1 Mitte Home-Leihgerät:
Dein Wasseraufbereiter
1 Mitte Balance-Kartusche
zum Filtern und Mineralisieren
1 CO₂-Zylinder
zum Sprudeln & besserer Aufnahme der Mineralien
Wasserflasche(n)
860 ml, aus Glas und/oder PET
Danach senden wir dir regelmäßig eine neue Kartusche zu – du bestimmst, wie oft.
CO₂ zum Sprudeln kannst du selbst je nach Bedarf in unserem Shop nachbestellen – immer rechtzeitig mit der Smart Refills-Funktion.
Nach Ende deines Abos sendest du uns das Gerät wieder zurück.
So funktioniert das Abo:
Zu Beginn schicken wir dir das Mitte Home-Starterkit. Es enthält:
1 Mitte Home-Leihgerät:
Dein Wasseraufbereiter
1 Mitte Balance-Kartusche
zum Filtern und Mineralisieren
1 CO₂-Zylinder
zum Sprudeln & besserer Aufnahme der Mineralien
Wasserflasche(n)
860 ml, aus Glas und/oder PET
Danach senden wir dir regelmäßig eine neue Kartusche zu – du bestimmst, wie oft.
CO₂ zum Sprudeln kannst du selbst je nach Bedarf in unserem Shop nachbestellen – immer rechtzeitig mit der Smart Refills-Funktion.
Nach Ende deines Abos sendest du uns das Gerät wieder zurück.
So funktioniert das Abo:
Zu Beginn schicken wir dir das Mitte Home-Starterkit. Es enthält:
1 Mitte Home-Leihgerät:
Dein Wasseraufbereiter
1 Mitte Balance-Kartusche
zum Filtern und Mineralisieren
1 CO₂-Zylinder
zum Sprudeln & besserer Aufnahme der Mineralien
Wasserflasche(n)
860 ml, aus Glas und/oder PET
Danach senden wir dir regelmäßig eine neue Kartusche zu – du bestimmst, wie oft.
CO₂ zum Sprudeln kannst du selbst je nach Bedarf in unserem Shop nachbestellen – immer rechtzeitig mit der Smart Refills-Funktion.
Nach Ende deines Abos sendest du uns das Gerät wieder zurück.
Teste einen Monat für nur 9,99 €
Dein Starterkit ist in 3-5 Werktage bei dir
Preisangaben pro Liter variieren je nach CO2-Verbrauch.
S-Abo
M-Abo
L-Abo
Business-Abo
Mitte Home: Das Gerät
Die Mitte-Kartusche
Der CO₂-Zylinder / Smart Refills

Registriere dich für unseren Newsletter für exklusive Angebote, Events und ganz viel Wasser-Wissen.
Häufige Fragen
Fällt eine Gebühr für die Nutzung der Mitte-App an?
Für die Nutzung der Mitte-App fällt keine Gebühr an. Du bezahlst nur für die Mitte Kartuschen und Mitte CO₂-Zylinder, die du bestellst.
Kann ich meine Kartuschenauswahl ändern?
Starter-Kits, die mit Mitte-Wasser-Abos geliefert werden, enthalten unsere Balance-Kartusche. Während des Abo-Zeitraums können Kunden ihre Kartuschen-Auswahl ändern. Bitte lesen Sie diesen Artikel für weitere Informationen: Kann ich meine Kartuschen-Auswahl ändern?
Wann wird mir die monatliche Abonnementgebühr in Rechnung gestellt?
Deine erste Gebühr wird mit deiner Bestellung abgebucht. Die nachfolgenden monatlichen Abo-Beiträge werden jeweils 1 Kalendermonat ab dem Lieferdatum deines Mitte Home-Starterkits berechnet.
Beispiel: Du kaufst das Mitte Wasser-Abo am 01.01.23 in unserem Shop, dann wird dir die erste Rate berechnet. Das Mitte Home Starter Kit wird dir am 04.01.23 geliefert. Dein nächster Monatsbeitrag wird am 04.02.23 abgebucht.
Was sind die Geschäftsbedingungen für Mitte-Wasser-Abos?
Welche Zahlungsmöglichkeiten gibt es für Wasser-Abos?
Das Mitte Wasser-Abo kann per Kreditkarte oder Paypal bezahlt werden.
Was sind die Mindesthaltbarkeitsdaten der Kartuschen
Alle Mitte Home-Kartuschen haben ein 180-tägiges Mindesthaltbarkeitsdatum ab der Kartuschenaktivierung. Kartuschen können auch nach diesen 180 Tagen weiter verwendet werden. Wir empfehlen sehr, die Kartusche innerhalb von 180 Tagen ab der Kartuschenaktivierung aufzubrauchen. Optimales Mineralisierungs- und Filtationsergebnisse können danach nicht mehr garantiert werden.
Das Mindesthaltbarkeitsdatum wird in der App unterhalb des Kartuschenstatus angezeigt.
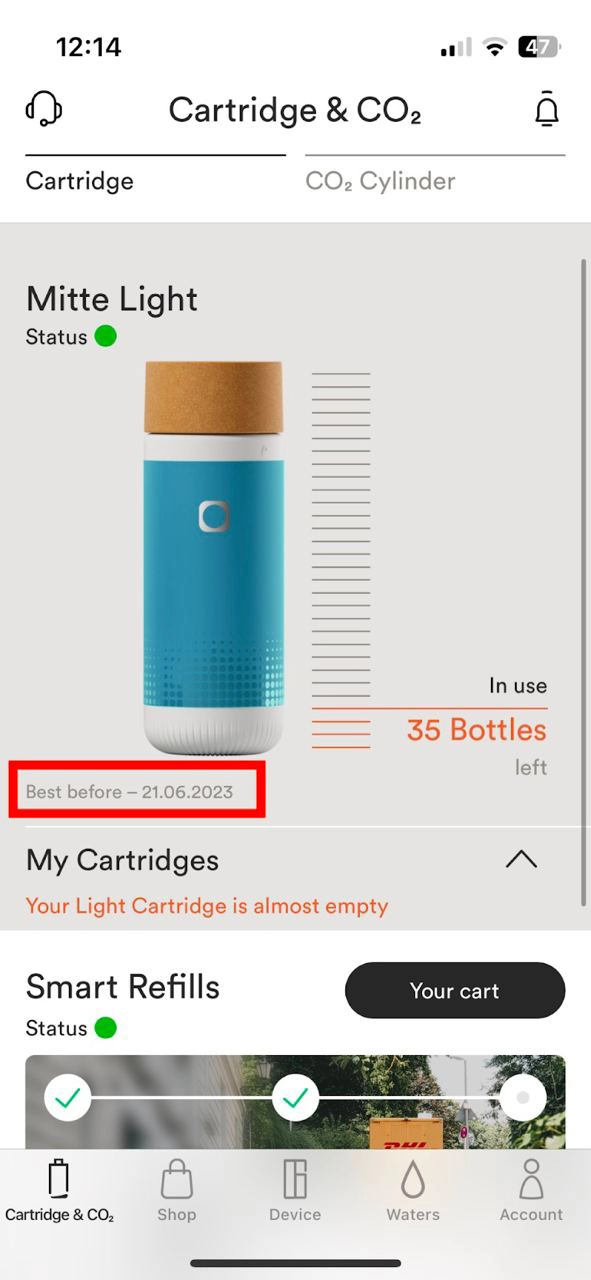
Wenn das Mindesthaltbarkeitsdatum überschritten ist und du eine Wasserauswahl triffst, wird die Azeigeleuchte auf dem Gerätkopf weiß-orange blinken sowie die Kartuschenanzeigeleuchte dreimal schnell aufleuchten.
Bitte beachte: Mit früheren Firmware-Versionen hatten Kartuschen ein Ablaufdatum. Mehr Informationen dazu findest du hier.
Welche Kündigungsbedingungen gelten für das Mitte Wasser-Abo?
Kunden können ihr Mitte Wasser-Abo jederzeit innerhalb der ersten 30 Tage kündigen. Die Gebühr für den ersten Monat des Abos wird nicht erstattet.
Nach Ablauf des 30-tägigen Probezeitraums gilt für Kunden die folgende Mindestvertragslaufzeit, abhängig vom ausgewählten Abo-Paket.








.webp)
.webp)
.webp)
.webp)
.webp)
.webp)